Abstract
Background. Short- and long-term lung damage after coronavirus disease 2019 (COVID-19) has been emphasized in many studies, but pulmonary-specific health-related quality of life (HRQOL) has been examined only in a limited capacity.
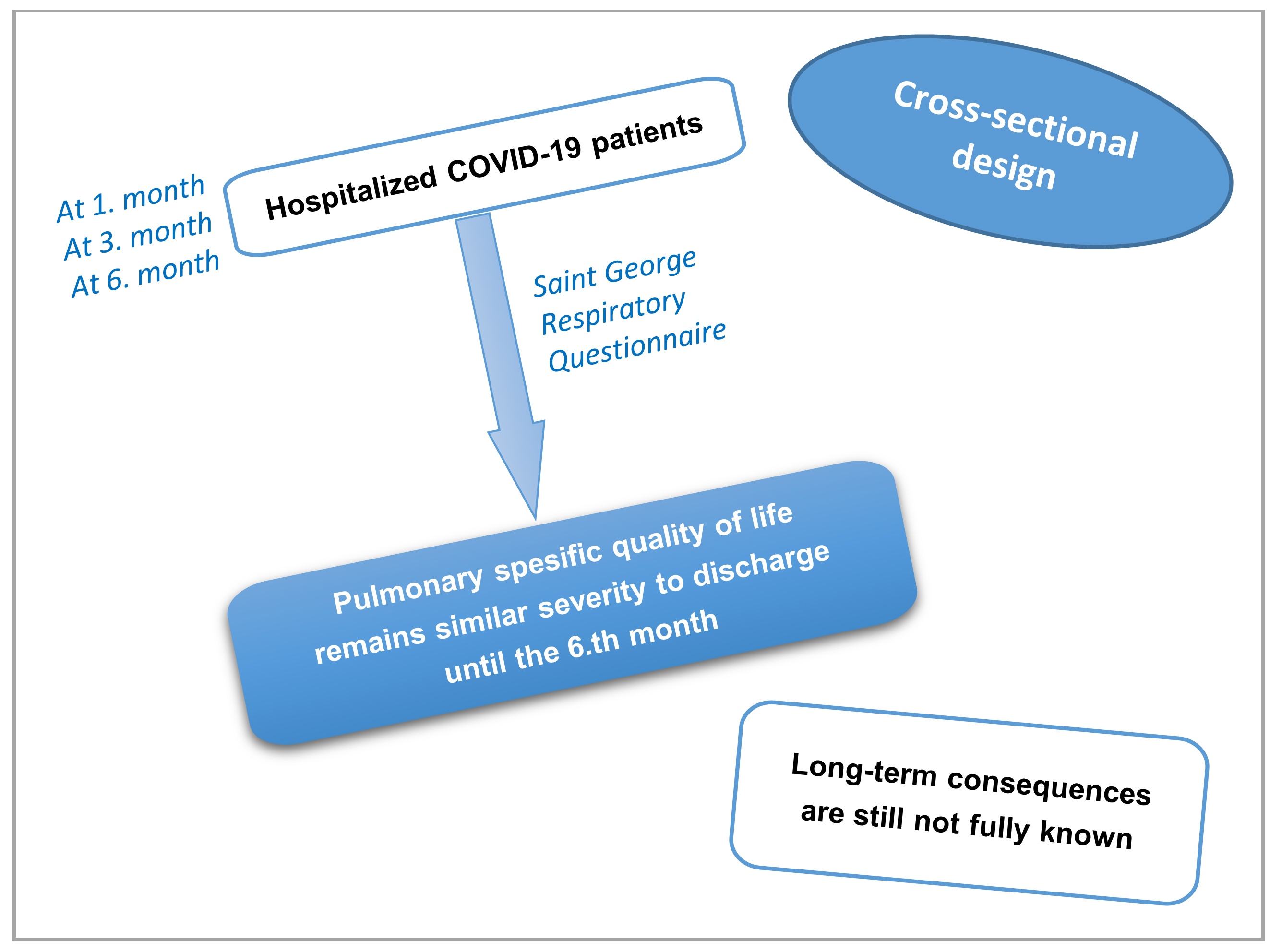
Objectives. In this study, we aimed to assess pulmonary-specific HRQOL and dyspnea among patients hospitalized for COVID-19 by applying the St George’s Respiratory Questionnaire (SGRQ) to patient groups 1, 3 and 6 months following discharge (groups T1, T3 and T6).
Materials and methods. This cross-sectional study was conducted between April 2020 and December 2020 at a tertiary hospital in Turkey. A total of 345 patients with a definite diagnosis of COVID-19 were included in our research.
Results. Total SGRQ score was significantly lower in the T6 group than in the T1 group (p < 0.001). The SGRQ-Symptom score was similar in the T3 and T6 groups, while the T1 group had significantly higher values (p < 0.001). The SGRQ-Activity score was significantly lower in the T6 group than in the T1 and T3 groups (p = 0.001), while the SGRQ-Impact score was significantly higher in the T6 group compared to the other 2 groups (p < 0.001). When the patients were analyzed statistically in terms of dyspnea, the difference between the baseline and 6-month results was found to be statistically significant (p < 0.001).
Conclusions. Although long-term consequences are still not fully known, the SGRQ scores and dyspnea outcomes of our patients show that pulmonary-specific HRQOL and dyspnea remain at a similar level from discharge until the 6th month after discharge. Studies with extended and longitudinal follow-up are required.
Key words: COVID-19, pulmonary-specific quality of life, St George’s Respiratory Questionnaire (SGRQ), dyspnea
Background
Coronavirus disease-2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), emerged in late 2019 and quickly spread to become a worldwide pandemic.1 As of August 22, 2022, a total of 593,269,262 people were diagnosed with the virus globally, resulting in 6,446,547 deaths.2 Previous studies have shown that COVID-19 affects multiple organs, although the lung is the main organ affected.3, 4 In addition to the effects of the disease on the pulmonary system in the acute phase, the long-term consequences on pulmonary functions and the extent to which they influence health-related quality of life (HRQOL) have been the subject of many studies.5, 6, 7 They showed the presence of chronic symptom burden and poor quality of life in COVID-19 survivors. These studies also show that COVID-19 can affect the HRQOL of patients, as well as healthcare employees and the general population.8, 9, 10, 11 A previous cross-sectional study revealed that COVID-19 patients had poor HRQOL at 1-month follow-up, which was influenced by various risk factors.12 Another study demonstrated a reduction in HRQOL 3 months after recovery from acute COVID-19.13
Many scales have been used to measure the impact of COVID-19 on HRQOL during active disease and follow-up periods.5, 6, 7, 14, 15 However, the majority of scales used assess overall HRQOL, and the number of studies measuring pulmonary-specific HRQOL is limited. The St George’s Respiratory Questionnaire (SGRQ) is a standardized, self-administered test that measures impacts on general health, daily living and perceived well-being in lung-specific chronic diseases such as chronic obstructive pulmonary disease, asthma, bronchiectasis, kyphoscoliosis, sarcoidosis, and cystic fibrosis.16, 17 There are currently very few studies in which SGRQ was applied in the follow-up of COVID-19 patients, and although they confirm that patients’ lung-specific HRQOL improved over time, the number of patients was low and follow-up periods were limited.6, 7, 18 Despite the emphasis on short- and long-term lung damage after COVID-19, little is known about the extent to which these sequelae affect pulmonary-specific HRQOL.
Objectives
This study aimed to determine changes observed over time regarding pulmonary-specific HRQOL and dyspnea symptoms in patients hospitalized for COVID-19 by assessing groups of patients 1, 3 and 6 months after discharge.
Materials and methods
Study design
This cross-sectional single-center study was conducted between April 2020 and December 2020 at the Sultan 2nd Abdulhamid Han Training and Research Hospital (Istanbul, Turkey), a pandemic hospital of the Health Sciences University (Istanbul, Turkey). The Ethics Committee of the Health Sciences University, Hamidiye Clinical Research Institute, approved the study (approval No. 18/7 issued on June 14, 2021). The study conformed to the ethical standards of the Declaration of Helsinki. All study participants provided written informed consent.
Study population
The study included 345 patients diagnosed with COVID-19 using positive SARS-CoV-2 polymerase chain reaction (PCR) test results from nasopharyngeal swab samples or with a conclusive diagnosis of COVID-19 according to computed tomography (CT) and clinical and laboratory findings, as recommended by the relevant guidelines of the World Health Organization (WHO). Other inclusion criteria included age >18 years and serious illness or symptoms caused by or suspected to have been caused by COVID-19 that required hospitalization (intensive care units (ICUs) or wards). Exclusion criteria were refusal to participate, advanced heart failure, deep anemia, advanced chest deformity, neuromuscular diseases that may cause respiratory distress, pregnancy, dialysis, malignancy, long-term immobility, and cognitive problems preventing scale administration.
Data collection
The hospital database identified patients who had passed the 1-month (T1), 3-month (T3) and 6-month (T6) thresholds of discharge (from the day of diagnosis). Participants were randomly selected using patient codes, and each was contacted by trained healthcare personnel via telephone to schedule appointments. A total of 1562 patients were contacted, and 345 randomly selected individuals (115 patients in each group) participated in the study. Data obtained from hospital records during the hospitalization period (inpatient wards or ICUs) included sociodemographic data, comorbidities, smoking status, day of admission after symptom onset, PCR test results, length of hospital stay, symptoms, dialysis requirement, intensive care need, type of respiratory support, and oxygen saturation.
Follow-up consultations with the patients took place in the COVID-19 outpatient clinic, which was established for the follow-up of COVID-19 patients in the Sultan 2nd Abdulhamid Han Training and Research Hospital. All participants were interviewed face-to-face by trained physicians, and provided the SGRQ themselves. Changes in the number of people who developed dyspnea were also evaluated.
The St George’s Respiratory Questionnaire scale and its administration
The SGRQ consists of 76 items divided into 3 sections, including symptoms (assessing respiratory symptoms, frequency and severity), activity (activities causing or limited due to dyspnea) and impacts (aspects associated with functioning and psychological disturbances resulting from respiratory illness). Each section and total score received scores ranging from 0 to 100 points.16, 19 The total score summarizes the overall impact of the illness on health status, with a higher score indicating worse health and 0 indicating best possible health in relation to pulmonary disease. A reduction of 4 units in the SGRQ score after a medical or non-medical intervention is generally accepted in the literature as a valid minimally important difference (MID) of beneficial therapy.17 The patients filled out the questionnaires while seated in a quiet room and after receiving advice on how and why they should fill in the questionnaire and answer all the questions. When the patient finished, the questionnaire was checked to ensure that all questions were answered, and items without a response were shown to the patient who completed it. The 3 SGRQ component scores and the total score were calculated using a Microsoft Excel 2016 spreadsheet (Microsoft Corp., Redmond, USA) called the SGRQ Calculator.
Statistical analyses
All analyses employed IBM SPSS v. 25 (IBM Corp., Armonk, USA) or Number Cruncher Statistical System (NCSS) 2020 Statistical Software (NCSS LLC, Kaysville, USA), with p < 0.05 accepted as statistically significant and 95% confidence intervals (95% CIs) calculated. Quantitative variables are reported as mean ± standard deviation (M ±SD), and qualitative variables as frequency, percentage and median (Q1–Q3 percentile values). The Shapiro–Wilk test and visual inspection of box plots were employed to evaluate data distribution. A comparison of the 2 groups utilized Student’s t-tests or Mann–Whitney U tests, as appropriate. Meanwhile, a one-way analysis of variance (ANOVA) was used to compare 3 groups, followed by a post hoc Bonferroni test, Kruskal–Wallis test or Dunn test, as appropriate. Wilcoxon signed rank test enabled intragroup evaluations, and the relationships between variables were evaluated using Pearson’s or Spearman’s correlation analysis, as appropriate. Further analysis used linear regression models. A comparison of qualitative data utilized a χ2 test with Yates’s correction for continuity or Fisher’s exact test, as appropriate.
Results
The study included 196 males and 149 females, with no significant difference between the groups in terms of sex distribution (p = 0.541). The overall mean age was 53.02 ±16.02 years, and the group means were 60.40 ±13.78 years (T1), 53.89 ±16.10 years (T3) and 44.76 ±16.66 years (T6). There was a significant difference in age between the groups (p < 0.001). While the percentages of patients with dyspnea in the T1 and T3 groups were similar, there was a statistically significant decrease in the percentage of patients with dyspnea in the T6 group (p < 0.001). Table 1 presents total and individual values and the differences between groups for comorbidities, smoking status, day of admission after symptom onset, PCR positivity, length of hospital stay, symptoms, dialysis need, intensive care need, oxygen saturation, and type of ventilation support.
Although the SGRQ-Total score was significantly lower in the T6 group than in the T1 group (p < 0.001), no significant difference was observed when the T3 group was compared to the other 2 groups. Meanwhile, the SGRQ-Symptom score was similar in the T3 and T6 groups, and significantly lower in both groups relative to the T1 group (p < 0.001). The SGRQ-Activity score was significantly lower in the T6 group than in the T1 and T3 groups (p = 0.001), while values were similar in the T1 and T3 groups. Likewise, the T1 and T3 groups had similar SGRQ-Impact scores, and the T6 group had significantly higher values compared to the other 2 groups (p < 0.001) (Table 2 and Figure 1).
A multiple linear regression analysis revealed that the unstandardized β coefficient for the SGRQ-Total score in the T6 group was 1.106 points lower than in the T1 group (p = 0.001), and in the T3 group it was 0.739 points lower than in the T1 group (p = 0.019) after adjusting for age, sex, chronic disease, smoking status, and PCR result. In addition, being female (β = 1.074) and having a chronic disease (β = 1.008) significantly increased the SGRQ-Total score (p < 0.01).
The unstandardized β coefficient for the SGRQ-Symptom score was 0.768 points lower in the T6 group than in the T1 group (p = 0.003), and in the T3 group it was 0.782 points lower than in the T1 group (p = 0.001), after adjusting for age, sex, chronic disease, smoking status, and PCR result. Also, being female (β = 0.504) significantly increased the SGRQ-Symptom score (p < 0.05).
The unstandardized β coefficient for the SGRQ-Activity score was 1432 points lower in the T6 group than in the T1 group (p = 0.004) after adjusting for age, sex, chronic disease, smoking status, and PCR result. Furthermore, being female (β = 1.572) and having a chronic disease (β = 1.528) significantly increased the SGRQ-Activity score (p < 0.01).
The unstandardized β coefficient for the SGRQ-Impact score was 1270 points lower in the T6 group than in the T1 group (p = 0.001), and in the T3, it was 0.951 points lower than in the T1 group (p = 0.008), after adjusting for age, sex, chronic disease, smoking status, and PCR result. Moreover, being female (β = 1.062) and having a chronic disease (β = 1.144) significantly increased the SGRQ-Impact score (p < 0.01) (Table 3).
Discussion
Although the primary goal of treating hospitalized COVID-19-infected patients is limiting mortality, it has become clear that these infections can have significant long-term effects. Thus, healthcare systems have begun to open clinics dedicated to diagnosing and treating symptoms that persist following COVID-19.20 The primary aim of this study was to show how the pulmonary-specific HRQOL of COVID-19 patients changed in the 1st, 3rd and 6th months after discharge. The secondary aim was to assess changes in the number of patients with dyspnea during this period. According to the results, the SGQR-Total, SGQR-Activity and SGQR-Impact domains of the SGRQ score decreased gradually from the 1st month to the 6th month. The SGRQ-Syptom score was similar in the T3 and T6 groups, while the T1 group had significantly higher values. A higher score means worse health and a score of 0 indicates the best possible health status regarding lung disease. The multiple regression analysis also confirmed these significant relationships. In addition, the number of patients with dyspnea decreased significantly after 6 months.
The rate and severity of long-term pulmonary complications after COVID-19 are currently unknown. Nonetheless, current research shows that there can be a variety of persistent respiratory symptoms several months after recovery from SARS-CoV-2 infection.21 The long-term effects of COVID-19 on HRQOL and mental wellbeing have been evaluated in several studies using various scales,4, 5, 6, 9, 15, 21, 22, 23 such as the SGRQ, which is a pulmonary-specific HRQOL scale used for the evaluation of diseases such as chronic obstructive pulmonary disease, asthma, bronchiectasis, kyphoscoliosis, sarcoidosis, and cystic fibrosis.16, 17 However, studies using pulmonary-specific HRQOL rating scales for COVID-19 are scarce. One such study used the SGRQ to assess patients with pneumonia and severe respiratory failure due to COVID-19 on the day they were discharged and on the 15th day after discharge, and found a significant decrease in all 3 domain scores and total scores when comparing results from day 15 to those from the day of discharge.6 Zhou et al. also used the SGRQ in a prospective cohort study in which they divided COVID-19 patients into severe/critical, mild/moderate, asymptomatic, and healthy control groups. The SGRQ evaluation performed 3 months after recovery showed that the impact score, symptom score, activity score, and total score increased as the severity of the disease increased. However, the study did not measure how the score changed over time.24 Likewise, another study showed that the adapted SGRQ (aSGRQ) improved in hospitalized patients 6–8 weeks after discharge compared to baseline, though scores were still worse than in the general population. The same study reported a significant association between the male sex and hospitalization with a reduced quality of life.25
In a study of healthcare workers in Wuhan, China, that presented results from patients 1 year after discharge, the median SGRQ-Total score was higher than that of healthy controls, and the SGRQ-Total score and all 3 subscale scores were significantly higher in the critical/severe disease group than in the mild/moderate disease group.26 The results of the current study support the findings of similar studies using SGRQ. Indeed, the total score, symptom score, activity score, and impact score in the T6 group were significantly better than the T1 group. Furthermore, there were no differences between the T3 and T1 groups for total, activity and impact scores. Also, symptom scores were lower in T3 than in T1 but similar to T6, which was confirmed using the multiple regression analysis. Considering the follow-ups for improvement in the SGRQ score, it is thought that the evaluations to be made at the 6th month will be the most appropriate response time to treatment, though these data should be supported by more comprehensive studies with more homogeneous patient groups and longitudinal assessments.
Dyspnea is a subjective symptom of respiratory distress, usually developing 7–8 days after the onset of COVID-19 symptoms, and is more common in patients with severe illness.6 Even 2–3 months after discharge, approx. 50% of patients who recover from COVID-19 may continue to complain of dyspnea at rest and during exercise or daily activities.6 Dyspnea is associated with reduced functional capacity and a lower HRQOL.27 Several studies reported dyspnea as the most common symptom observed during follow-up after COVID-19.28 In a prospective study, persistent dyspnea was common and reported by 58.4% of patients 1 year after discharge from ICU.28 In another prospective study, dyspnea was reported in 54% of the participants 3 months after discharge, although there was no difference between patients admitted to the ICU and those who were not.14 In our study, dyspnea was the most common initial symptom. However, there was no significant change in the number of patients with dyspnea in the T3 group compared to the T1 group. Although complaints of dyspnea did not return to a normal level for a given population, there were significant decreases in this regard after 6 months. Therefore, longer follow-up studies are required to understand the mechanisms of persistent dyspnea in survivors and to improve patient management after COVID-19.
Limitations of the study
Patients included in the three-time periods were not the same individuals, but different, which was considered a limitation of our study. The most important reason for this was the necessity of face-to-face interviews with the patients since the validity of administering the SGRQ questionnaire via other means has not been confirmed.16 Another limitation was the inevitable consequence of heterogeneity between patient groups. Although many differences between the groups, including age, seem to be a limitation, this disadvantage was minimized by the multiple regression analysis adjusted for parameters such as age, sex, chronic disease, smoking status, and PCR result. In addition, there was no difference between the groups in terms of pulmonary comorbidity.
A control group was not included due to the difficulty in conducting the questionnaire and the nature of the study. Furthermore, a control group was deemed unnecessary based on the evident differences between controls and patients with COVID-19. Although the follow-up period was relatively longer than in previous studies, the reversibility of pulmonary injury in the longer term is a matter of debate, and further follow-up could be necessary to elucidate possible improvements.
The laboratory and radiological findings and treatment characteristics that may affect the outcome were not specified in the groups. Indeed, viral load may be an important marker for the prognosis of COVID-19, but it was not examined in this study.
Advantages of this study over similar studies include a 3-stage follow-up period evaluated, which allowed for the assessment of gradual changes in pulmonary-specific HRQOL. Furthermore, the number of participants was higher than in most of similar studies. Nonetheless, studies with a larger patient count, longer follow-up periods, more homogeneous patient groups, and more detailed data are needed to confirm these results.
Conclusions
In conclusion, this study found that the SGRQ-Total, -Activity and -Impact scores of patients discharged after COVID-19 were lower in the T6 group compared to the T1 and T3 groups. Of note, the T6 and T3 groups demonstrated significantly better results compared to the T1 group in terms of SGRQ-Symptom scores. There was also a significant improvement in pulmonary-specific HRQOL and dyspnea complaints in the T6 group. Studies on the effects of COVID-19 on pulmonary-specific HRQOL are important to allow for a better preparation for future outbreaks caused by new variants of SARS-CoV-2 or other microorganisms. Furthermore, studies with a more homogeneous distribution of patient characteristics that employ long-term follow-up are required to assess and appropriately manage persistent lung injury in COVID-19.
Supplementary materials
The supplementary files are available at https://doi.org/10.5281/zenodo.7742320. The package contains the following files:
Supplementary Table 1. Normal distribution.
Supplementary Table 2. Predicting SGRQ-Total score.
Supplementary Table 3. Predicting SGRQ-Activity score.
Supplementary Table 4. Predicting SGRQ-Symptom score.
Supplementary Table 5. Predicting SGRQ-Impact score.
Supplementary Fig. 1. Distribution of SGRQ-Total scores.
Supplementary Fig. 2. Distribution of SGRQ-Symptom scores.
Supplementary Fig. 3. Distribution of SGRQ-Activity scores.
Supplementary Fig. 4. Distribution of SGRQ-Impact scores.
Supplementary Fig. 5. SGRQ-Total score distribution Q-Q plots.
Supplementary Fig. 6. SGRQ-Symptom score distribution Q-Q plots.
Supplementary Fig. 7. SGRQ-Activity score distribution Q-Q plots.
Supplementary Fig. 8. SGRQ-Impact score distribution Q-Q plots.
Supplementary Fig. 9. SGRQ-Total score detrended normal Q-Q plots.
Supplementary Fig. 10. SGRQ-Symptom score detrended normal Q-Q plots.
Supplementary Fig. 11. SGRQ-Activity score detrended normal Q-Q plots.
Supplementary Fig. 12. SGRQ-Impact score detrended normal Q-Q plots.
Supplementary Fig. 13. Box plot of SGRQ-Total score and sub-factors.